)CT205菌株固体发酵及防控草莓根腐病的研究
沈婷, 张园园, 王辰, 王世梅. 2015. 白刺链霉菌(Streptomyces albospinus)CT205菌株固体发酵及防控草莓根腐病的研究[J]. 南京农业大学学报, 38(4): 596-601.

SHEN Ting, ZHANG Yuanyuan, WANG Chen, WANG Shimei. Study on solid fermentation of Streptomyces albospinus CT205 and biocontrol effect against strawberry root rot[J]. Journal of Nanjing Agricultural University, 38(4): 596-601. DOI: 10.7685/j.issn.1000-2030.2015.04.011

白刺链霉菌(Streptomyces albospinus)CT205菌株固体发酵及防控草莓根腐病的研究
沈婷,
张园园,
王辰,
王世梅

南京农业大学资源与环境科学学院/江苏省固体有机废弃物资源化高技术研究重点实验室, 江苏 南京 210095
收稿日期:2014-11-17
基金项目:江苏省科技支撑计划项目(BE2013427)
作者简介:沈婷,硕士研究生。
通信作者:王世梅,教授,主要从事微生物发酵及土传病害生物防控,E-mail:smwang@njau.edu.cn。
摘要:[目的]为了明确草莓根腐病的致病因子,降低其发病率,从草莓病株根部分离病原菌,并研究拮抗菌白刺链霉菌(Streptomyces albospinus) CT205固体制剂对草莓根腐病的防控效果。[方法]从草莓病株根部分离出了1株病原菌Y3,采用形态观察及rDNA-ITS序列分析对病原菌Y3进行鉴定,用平板对峙培养测定拮抗菌CT205对菌株Y3的抑制作用,利用最佳固体发酵培养基及发酵条件对拮抗菌CT205进行固体发酵,通过连作致病土盆栽试验评价菌株CT205固体制剂对草莓根腐病的防控作用。[结果]初步鉴定病原菌Y3为尖孢镰刀菌(Fusarium sp.)。试验发现拮抗菌CT205对病原菌Y3的抑制作用较强,CT205固体发酵6 d,其制剂中菌含量达到2.2×109 CFU·g-1。盆栽试验表明,施用CT205固体菌剂对草莓生长有一定的促生作用,对草莓根腐病的防治效果为61.18%。[结论]采用拮抗菌CT205固体制剂防控草莓根腐病具有较高的潜在应用价值。
关键词: Streptomyces albospinus CT205 固体发酵 草莓根腐病 鉴定 生防效果
Study on solid fermentation of Streptomyces albospinus CT205 and biocontrol effect against strawberry root rot
SHEN Ting,
ZHANG Yuanyuan,
WANG Chen,
WANG Shimei

College of Resources and Environmental Sciences/Jiangsu Province Key Laboratory of Solid Organic Waste Utilization, Nanjing Agricultural University, Nanjing 210095, China
Abstract: [Objectives]In order to verify the causing factor of strawberry root rot and reduce the disease incidence,author tried to separate pathogenic fungus from strawberry root,and to study the effect of solid fermentation preparation of antagonistic Streptomyces albospinus CT205,separated from soil in early stage,against strawberry root rot. [Methods]The pathogenic Y3,separated from strawberry root with tissue separating methods,was identified according to morphological characteristics observed by optical microscope and rDNA-ITS sequences analysis. The PDA agar plate method was used to determine antifungal activity of S.albospinus CT205 against strain Y3. Strain CT205 was fermented with the optimized solid fermentation medium and fermentation conditions,and a pot experiment was conducted to evaluate the control efficiency of strain CT205 solid fermentation preparation against strawberry root rot with pathogenic soil from a field that had been cultivated with strawberry consecutively for several years. [Results]Strain Y3 was tentatively identified as Fusarium sp.,and the pathogenicity of strain Y3 was validated with the Koch rule. The experiment showed that strain CT205 exhibited strong antagonistic effect on mycelium growth of test pathogen Y3. And it was discovered that the mycelium of strain Y3 deformation distorted with the presence of strain CT205 by microscopic examination. Strain CT205 was fermented for six days at 28 ℃ with the optimal medium,which was 500 g·kg-1 wheat bran,120 g·kg-1 millet flour,60 g·kg-1 soybean meal,120 g·kg-1 rice bran,200 g·kg-1 rice hull,3 g·kg-1 CaCO3,the ratio of material to water 1:0.8,pH 7.5. At the end of the fermentation,the viable count was measured,and it was found that the amount of the spores reached 2.2×109 CFU·g-1. The pot experiment showed that it promoted somewhat the growth of strawberry after using 1% solid fermentation preparation of CT205,besides,the control efficiency against root rot of strawberry reached as high as 61.18%. [Conclusions]The solid fermentation preparation of S.albospinus CT205 has better potential for the control of strawberry root rot. But there are some differences between pot experiment and field experiment,so the actual control effect is still needed to validate with the field test.
Keywords: Streptomyces albospinus CT205 solid fermentation strawberry root rot identification bio-control effect
草莓连作障碍在草莓种植区普遍存在,对草莓种植业危害严重,而根腐病是草莓连作障碍中最主要的病害之一,已成为制约草莓产业快速发展的主要障碍[]。目前,报道的最常见的引起草莓根腐病病原菌有立枯丝核菌(Rhizoctonia solani)、尖孢镰刀菌(Fusarium oxysporum)、疫霉(Phytoqhthora fragariae)及大丽轮枝菌(Verticillium dahliae)等[, ]。农业生产上防治草莓根腐病一般采用轮作[]、土壤消毒[]等措施。轮作效果较好,但难以适应集约化设施农业种植模式的要求;利用溴甲烷等化学药剂对土壤进行消毒,可有效降低草莓根腐病的发病率,但对土壤环境污染较大,易产生药物残留等问题,并且已被世界大多数国家禁止使用[]。因此,寻找更安全高效的生防制剂防治草莓根腐病具有重要的意义。
近年来,国内外科技工作者对草莓根腐病的生物防治已有研究报道,已分离获得有一定生防效果的细菌、放线菌及真菌[, , ]。徐淑华等[]从草莓种植土壤中筛选出1株生防细菌,盆栽试验显示其对草莓根腐病的防治效果为51.75%,并能显著促进草莓的生长发育;王占武等[]研究发现枯草芽孢杆菌B501对草莓枯萎病(Fusaruim oxysporum f.sp.fragriae)有广谱杀菌的作用,而且也能够很好地在草莓根际定殖;申光辉等[]从草莓根域土壤中筛选出了对草莓根腐病有拮抗作用的真菌HF3和HF7,盆栽试验表明其对草莓有很好的促生效果,能有效地提高草莓的生物量,对根腐病的防效为53.0%和46.9%。目前,生物防治植物病害最传统的做法是将生防菌制成活菌制剂直接应用,但液体制剂存在不易保存、活性不稳定等问题。而通过固体发酵生产的活菌菌剂,其孢子数量大,活性强,便于贮藏和运输,且发酵设备简单,成本低廉,易于在生产中推广应用[]。本实验室前期筛选到1株生防效果优良的白刺链霉菌(Streptomyces albospinus)CT205,对多种植物病原真菌均有较强的抑制作用[]。本研究尝试从草莓病株根茎部位分离出高致病的病原菌,对其进行初步鉴定及柯赫氏法则验证,进而研究拮抗菌CT205的固体发酵制剂对草莓根腐病的防控效果,以期为放线菌固体发酵制剂用于草莓连作障碍的修复提供科学依据。
1 材料与方法 1.1 供试菌株Streptomyces albospinus CT205由本实验室分离保存。
1.2 供试培养基马铃薯葡萄糖琼脂培养基(PDA培养基):马铃薯200 g,葡萄糖20 g,琼脂20 g,蒸馏水1 L,pH自然。
马丁氏培养基:葡萄糖10 g,蛋白胨5 g,KH2PO4 1 g,MgSO4 · 7H2O 0.5 g,孟加拉红30 mg,琼脂20 g,蒸馏水1 L,pH自然,115 ℃灭菌30 min后加入0.03 mg链霉素。
CT205种子培养基:葡萄糖45 g,黄豆粉30 g,酵母粉5 g,CaCO3 5 g,蒸馏水1 L,pH 7.5,121 ℃灭菌20 min。
CT205固体基础发酵培养基:麦麸500 g · kg-1,小米粉120 g · kg-1,黄豆粉60 g · kg-1,米糠120 g · kg-1,稻壳200 g · kg-1,CaCO3 3 g · kg-1,料和水质量比1 ∶ 0.8,起始pH 7.5,121 ℃灭菌20 min。
1.3 草莓根腐病病原菌的分离鉴定 1.3.1 病原菌Y3的分离从南京麒麟镇后村草莓种植大棚中,采集草莓根腐病发病植株,采用常规组织分离法[],先用自来水冲洗植株根系表面泥沙,再用吸水纸吸去表面多余水分,剪去须根,在病健交界处切取适当大小的组织块,用75%(体积分数)乙醇表面消毒30 s,再用0.1%(体积分数)升汞浸泡1 min,最后用无菌水浸洗3~4次,置于马丁氏培养基上30 ℃恒温培养4~5 d,挑取病斑组织边缘长出的菌丝接种于PDA培养基上进行纯化。
1.3.2 病原菌Y3的形态特征观察观察、记录病原菌Y3的菌落形态和气生菌丝生长状况等培养特征。显微镜下观察分生孢子形态等形态特征。
1.3.3 病原菌Y3基因组DNA的提取及rDNA-ITS的PCR扩增采用OMEGA公司的试剂盒提取菌株基因组DNA,真菌通用引物ITS1(5′-AGAAGTCGTAACAAGGTTTCCGTAGG-3′)和ITS4(5′-TCCTCCGCTTATTGATATGC-3′)进行rDNA-ITSPCR扩增,PCR扩增产物由上海美吉有限公司进行纯化并测序,测序结果登录GenBank数据库,利用BLAST()与GenBank数据库中的序列进行比对,获得最相近菌株rDNA-ITS序列,采用MEGA 4.0软件绘制系统发育树。
1.4 病原菌柯赫氏法则验证采用伤根法接种菌株Y3的孢子悬液在健康草莓的根系周围,观察发病症状,从发病植物根部分离病原菌,并进行初步鉴定。
1.5 菌株CT205对病原菌Y3的拮抗作用采用平板对峙法,将培养好的病原菌Y3及生防菌CT205用打孔器(Φ=5 mm)打块,将其分别置于PDA平板上,对峙培养,30 ℃培养5~7 d,观察抑菌效果。
1.6 菌株CT205固体发酵 1.6.1 CT205种子液的制备菌株CT205在PDA培养基斜面上活化,28 ℃培养5~7 d,待分生孢子丰满,挑取3环孢子,接种于50 mL种子培养基中(装于250 mL三角瓶),30 ℃、170 r · min-1振荡培养48 h,备用。
1.6.2 CT205固体发酵在250 mL三角瓶中,装入20 g固体基础发酵培养基,料和水质量比为1 ∶ 0.8,121 ℃灭菌20 min。接种15%(体积分数)的种子液,28 ℃培养6 d,每天用无菌玻璃棒翻动2次混匀。发酵结束后,用稀释涂布法测定制剂中菌含量。
1.7 草莓盆栽试验 1.7.1 盆栽试验设计选取长势较好且大小一致的健康草莓幼苗(红颊),移栽到装有1.5 kg草莓连作土的盆钵中,每盆1株,常规管理。试验设3个处理:固体发酵菌剂处理、菌悬液处理和对照。固体发酵菌剂处理为1%(质量分数)固体发酵菌剂(15 g)提前与盆栽土混合拌匀装入盆钵中,菌悬液处理为1%(体积分数)菌悬液(15 mL)用水稀释后沿根灌注。对照为草莓连作土,不加任何处理。病原菌Y3用PDA液体培养基摇瓶培养5 d后,收获分生孢子并调整其孢子浓度为105 mL-1。在草莓移栽缓苗1周后,采用灌注法接种在草莓根系周围,接种量为每盆5 mL,每个处理10盆。于2014年3月14日进行盆栽试验,定期观察记录草莓生长情况,2个月后结束盆栽试验,挖取植株,统计发病程度及生物量。
1.7.2 病情指数测定草莓根腐病发病程度采用Vestberg等[]的方法分为6级:0级为根系未发病;1级为根系发病率小于等于30%,叶片正常;2级为根系发病率大于30%小于等于60%,叶片正常;3级为根系发病率大于60%小于等于80%,叶片变黄;4级为根系发病率80%以上,叶片枯萎;5级为整株死亡,叶片干枯。病情指数[]和生防效果分别按照下列公式计算。
病情指数=[∑(病级株数×代表数值)/(株数总和×发病最重级的代表数值)]×100%;
生防效果=(对照病情指数-处理病情指数)/对照病情指数×100%。
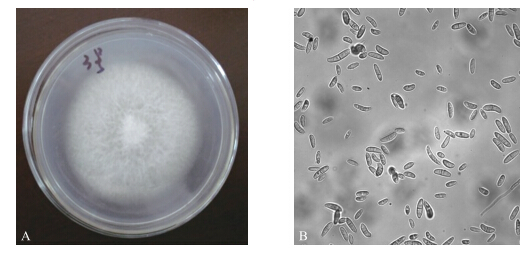
2 结果与分析 2.1 病原菌Y3的初步鉴定 2.1.1 病原菌Y3培养特征与形态特征观察菌株Y3在PDA培养基上30 ℃培养7 d后,菌落特征及分生孢子形态如图 1所示。Y3菌落正面中心脐状突起,有细微同心环纹,气生菌丝茂盛,絮状,菌落白色,随着培养时间的延长,基内菌丝分泌褐色色素。在光学显微镜下观察其气生菌丝及分生孢子形态发现,Y3菌丝分隔,分化形成的分生孢子呈圆柱状或镰刀状,稍弯。
2.1.2 病原菌Y3分子生物学鉴定
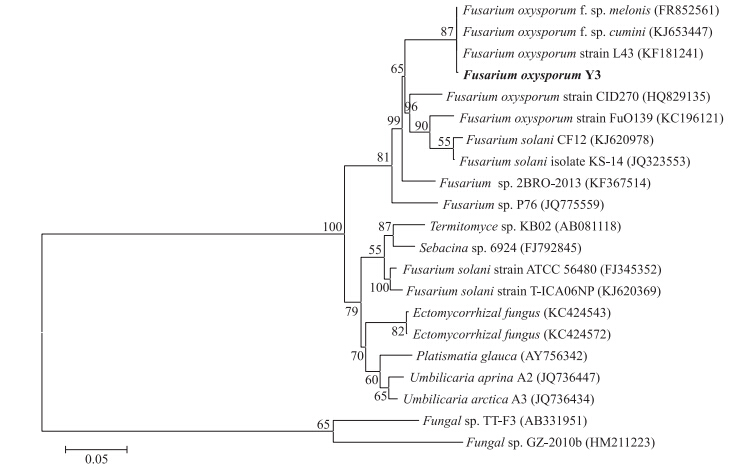
提取菌株Y3的基因组rDNA,扩增其rDNA-ITS序列,得到1条525 bp 左右的条带,在GenBank上进行同源性比对,发现与其一致性较高的菌株均属于镰刀菌属(Fusarium),与多株尖孢镰刀菌(F.oxysporum)的同源性为97%,根据菌株的形态特征并结合rDNA-ITS序列分析,将菌株Y3初步鉴定为尖孢镰刀菌,构建的系统发育树见图 2。
2.2 病原菌Y3柯赫氏法则验证
通过致病性回接试验发现,病原菌Y3接种3周后草莓发病,植株中柱变红,并且能够从接种发病的草莓植株根部重新分离得到该病菌,符合柯赫氏法则,证明其是草莓根腐病的病原菌。
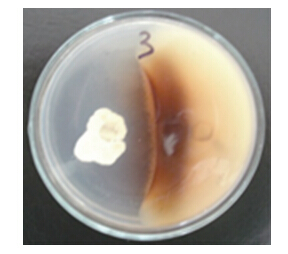
2.3 拮抗菌CT205对病原菌Y3的拮抗作用从图 3可以看出:菌株CT205对病原菌Y3有较强的抑制作用。病原菌Y3与拮抗菌CT205对峙培养后,在显微镜下观察到Y3菌丝体中的分隔增多,并出现明显扭曲及褶皱现象。
2.4 拮抗菌CT205固体发酵
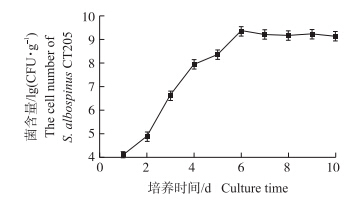
拮抗菌CT205在固体基础发酵培养基中发酵,接种3 d后,菌丝在基质中旺盛生长,瓶壁及基质布满白色的菌丝体,并散发出放线菌特有的“土腥味”,发酵6 d后,镜检发现大量的分生孢子,菌含量可达到2.2×109 CFU · g-1,之后略有波动,涨幅不大(图 4)。因此,确定固体发酵的终点时间为6 d,发酵结束后测定制剂的含水量为57%。S.albospinus CT205固体发酵制剂形态见图 5。

2.5 草莓盆栽试验
施用CT205固体发酵菌剂和CT205菌悬液处理与对照相比,茎叶鲜质量分别增加21.11%和12.21%,根鲜质量分别增加42.36%和32.76%,说明菌种CT205对草莓生长有一定的促生作用,且固体发酵菌剂的促生作用更明显。CT205固体发酵菌剂与CT205菌悬液处理中,草莓病情指数均明显下降,生防效果达到61.18%和55.22%(表 1)。
表 1 施用CT205制剂处理对草莓生长的影响(x±SD) Table 1 Effect of applying CT205 preparation on plant growth of strawberry
处理
Treatment 茎叶鲜质量/g
Shoot fresh weight 根鲜质量/g
Root fresh weight 病情指数/%
Disease index 生防效果/%
Control efficiency
对照CK 12.79±0.67c 4.06±0.41b 53.24 —
菌悬液Liquid agents 14.29±1.81ab 5.39±0.14ab 23.84 55.22
固体发酵菌剂Solid agents 15.49±0.54a 5.78±0.11a 20.67 61.18
3 结论与讨论
草莓根腐病主要症状是中柱发红,由内向外腐烂,后期植株全株萎焉枯死,是一种难以防治的根部毁灭性病害[]。笔者从草莓病株分离出多株病原真菌,经回接试验验证,1株致病性最强,编号为Y3,故选择Y3为研究材料。经形态观察和分子生物学鉴定,初步鉴定其为尖孢镰刀菌属。
放线菌具有适应环境能力强,在植物根表和根围土壤中有较强的定殖能力,能产生抗生素,分泌多种胞外水解酶,能产生丰富的分生孢子,且孢子存活时间长,有利于工业化生产及应用等优点,故研制放线菌生防制剂具有更重要的意义。拮抗菌CT205是本课题组前期筛选到的1株生防效果优良的放线菌,对多种植物病原真菌均有较强的抑制作用。张园园[]采用单因素试验确定了CT205固体发酵最合适的碳源、氮源以及无机盐组合,并在此基础上,运用L16(45)正交设计优化了固体发酵条件(如最佳接种量、料水比、起始pH、发酵温度和发酵终点等)。本研究采用其优化好的培养基配方及发酵条件,进行CT205固体发酵,发酵6 d,镜检发现大量的分生孢子,活菌计数菌含量达到2.2×109 CFU · g-1。
目前国内外用于草莓根腐病的生防菌有芽孢杆菌[]、木霉[, ]、丛枝菌根(AM)真菌[, ]等,亦有较多用放线菌制剂克服草莓连作障碍的报道[, , ],但尚未有用白刺链霉菌防治草莓根腐病的研究报道,本试验将白刺链霉菌CT205作为生防菌,为草莓根腐病害生物防控提供新的生防材料。生防菌剂能够保护植物根系免受病原菌侵染,其作用机制包括产生特有的抗生素,抑制病原菌菌丝生长,与病原菌竞争生存空间与营养物质,同时活菌制剂接入土壤可调控根际微生物区系,减少病原菌的数量,提高土壤对土传病原真菌的抑制能力,进而促进作物根系生长,提高抗病能力。后续将对CT205的作用机制进行深入研究。本试验研究CT205对草莓根腐病的防病促生作用是在盆栽条件下进行,盆栽试验表明CT205制剂对草莓根腐病有较好的防控作用,但盆栽试验与大田试验存在一定差异,故其实际的生防效果仍需田间试验验证。
参考文献(References)
Fang X L,Phillips D,Li H,et al. Comparisons of virulence of pathogens associated with crown and root diseases of strawberry in western Australia with special reference to the effect of temperature[J]. Scientia Horticulturae,2011,131:39-48
陈瑶,王树雪,魏艳敏,等. 草莓根腐病菌C16-4的分离鉴定及生物学特性研究[J]. 果树学报,2012,29(4):638-643 [Chen Y,Wang S X,Wei Y M,et al. Studies on isolation,identification and biological characteristics of pathogenic fungus strain C16-4 of strawberry root rot[J]. Journal of Fruit Science,2012,29(4):638-643(in Chinese with English abstract)]
Chamorro M,Miranda L,Domínguez P,et al. Evaluation of biosolarization for the control of charcoal rot disease(Macrophomina phaseolina)in strawberry[J]. Crop Protection,2015,60:279-286
Avilés M,Castillo S,Bascon J,et al. First report of Macrophomina phaseolina causing crown and root rot of strawberry in Spain[J]. Plant Pathology,2008,57:382
Benlioglu S,Boz O,Yildiz A,et al. Alternative soil solarization treatments for the control of soil-borne diseases and weeds of strawberry in the western Anatolia of Turkey[J]. Journal of Phytopathology,2005,153(7/8):423-430
Belova A,Narayan T,Olkin I. Methyl bromide alternatives for strawberry and tomato pre-plant uses:a meta-analysis[J]. Crop Protection,2013,54:1-14
Dias A C F,Costa F E C,Andreote F D,et al. Isolation of micropropagated strawberry endophytic bacteria and assessment of their potential for plant growth promotion[J]. World Journal of Microbiology and Biotechnology,2009,25(2):189-195
孙敬祖,薛泉宏,唐明,等. 放线菌制剂对连作草莓根区微生物区系的影响及其防病促生作用[J]. 西北农林科技大学学报:自然科学版,2009,37(12):153-158 [Sun J Z,Xue Q H,Tang M,et al. Study on the effect of actinomycetes on microflora of replanted strawberry's root domain and the bio-control effectiveness[J]. Journal of Northwest A&F University:Natural Science Edition,2009,37(12):153-158(in Chinese with English abstract)]
Kakvan N,Heydari A,Zamanizadeh H R,et al. Development of new bioformulations using Trichoderma and Talaromyces fungal antagonists for biological control of sugar beet damping-off disease[J]. Crop Protection,2013,53:80-84
徐淑华,蒋继志,姚克文,等. 两株拮抗细菌对草莓根腐病的抑制作用[J]. 河北农业大学学报,2005,28(3):81-83 [Xu S H,Jiang J Z,Yao K W,et al. Inhibition effect of two strains of antagonistic bacteria against Pestalotiops photiniae[J]. Journal of Hebei Agricultural University,2005,28(3):81-83(in Chinese with English abstract)]
王占武,李晓芝,刘彦利,等. 枯草芽孢杆菌B501在草莓根际的定殖及其动态变化[J]. 植物病理学报,2003,33(2):188-189 [Wang Z W,Li X Z,Liu Y L,et al. Colonization and population dynamics of Bacillus subtilis B501 in the rhizosphere of strawberry[J]. Acta Phytopathologica Sinica,2003,33(2):188-189(in Chinese with English abstract)]
申光辉,薛泉宏,张晶,等. 草莓根腐病拮抗真菌筛选鉴定及其防病促生作用[J]. 中国农业科学,2012,45(22):4612-4626 [Shen G H,Xue Q H,Zhang J,et al. Screening,identification and bio-control potential of antagonistic fungi against strawberry root rot and plant growth promotion[J]. Scientia Agricultura Sinica,2012,45(22):4612-4626(in Chinese with English abstract)]
田晓丽,赵红杰,唐彩乐,等. 生防放线菌153固态发酵条件的优化及其耐热力检测[J]. 西北农林科技大学学报:自然科学版,2010,38(7):181-186 [Tian X L,Zhao H J,Tang C L,et al. Optimization of the fermentation of solid state medium for bio-control actinomycetes 153 and its heat tolerance ability[J]. Journal of Northwest A&F University:Natural Science Edition,2010,38(7):181-186(in Chinese with English abstract)]
梁银,张谷月,王辰,等. 一株拮抗放线菌的鉴定及其对黄瓜枯萎病的生防效应研究[J]. 土壤学报,2013,50(4):174-180 [Liang Y,Zhang G Y,Wang C,et al. Identification and bio-control effect of a strain of actinomycete antagonistic to wilt disease of cucumber[J]. Acta Pedologica Sinica,2013,50(4):174-180(in Chinese with English abstract)]
方中达. 植病研究方法[M]. 北京:中国农业出版社,1998 [Fang Z D. Plant Pathology and Entomology Research Methods[M]. Beijing:China Agriculture Press,1998:122-126(in Chinese)]
Vestberg M,Kukkonen S,Saari K,et al. Microbial inoculation for improving the growth and health of micropropagated strawberry[J]. Applied Soil Ecology,2004,27(3):243-258
向发云,韩永超,曾祥国,等. 湖北省草莓育苗期炭疽病病害调查[J]. 湖北农业科学,2012,51(24):5651-5653 [Xiang F Y,Han Y C,Zeng X G,et al. A survey on the strawberry anthracnose disease in Hubei Province[J]. Hubei Agricultural Sciences,2012,51(24):5651-5653(In Chinese with English abstract)]
Manici L M,Caputo F,Baruzzi G. Additional experiences to elucidate the microbial component of soil suppressiveness towards strawberry black root rot complex[J]. Annals of Applied Biology,2005,146(4):421-431
张圆圆. 白刺链霉菌CT205固体发酵条件优化及其应用初探[D]. 南京:南京农业大学,2014:21-27 [Zhang Y Y. Optimization of solid state fermention conditions of Streptomyces albospinus CT205 and its preliminary application[D]. Nanjing:Nanjing Agricultural University,2014:21-27(in Chinese with English abstract)]
杜安楠,马跃,李贺,等. 木霉菌剂对草莓微繁苗生长和抗病性的影响[J]. 中国果树,2009(3):20-23 [Du A N,Ma Y,Li H,et al. Trichoderma micropropagation of strawberry seedlings and disease resistance[J]. China Fruit,2009(3):20-23(in Chinese with English abstract)]
张雪,张志宏,刘月学,等. 木霉菌剂提高'红颜’草莓炭疽病抗性的效应[J]. 西北农业学报,2010,19(8):153-156 [Zhang X,Zhang Z H,Liu Y X,et al. Effect of Trichodermaon improving resistance of strawberry to anthracnose[J]. Acta Agriculturae Boreali-occidentalis Sinica,2010,19(8):153-156(in Chinese with English abstract)]
Tahmatsidou V,O'Sullivan J,Cassells A C,et al. Comparison of AMF and PGPR inoculants for the suppression of Verticillium wilt of strawberry(Fragaria×ananassa cv.Selva)[J]. Applied Soil Ecology,2006,32(3):316-324
李明月,常敏,张庆华,等. 木榄内生真菌菌株ZD6及其代谢产物的抑菌活性[J]. 菌物学报,2010,29(5):739-745 [Li M Y,Chang M,Zhang Q H,et al. The endophytic fungus strain ZD6 isolated from the stem of Bruguiera gymnorrhiza and the antibacterial activity of its metabolites[J]. Mycosystema,2010,29(5):739-745(in Chinese with English abstract)]
陈宏州,庄义庆,杨敬辉. 黄麻链霉菌NF0919菌株对草莓枯萎病的生防活性初探[J]. 江西农业学报,2014,26(11):54-57 [Chen H Z,Zhuang Y Q,Yang J H. A primary study on biocontrol activity of Streptomyces corchorusii strain NF0919 to strawberry Fusarium wilt[J]. Acta Agriculturae Jiangxi,2014,26(11):54-57(in Chinese with English abstract)]
赤国彤,李亚宁,李星,等. 链霉菌水剂对草莓连作障碍的控制效果[J]. 贵州农业科学,2012,40(3):127-129 [Chi G T,Li Y N,Li X,et al. Control effects of Streptomyces as on replant disease of strawberry[J]. Guizhou Agricultural Sciences,2012,40(3):127-129(in Chinese with English abstract)]